What factors encourage the development of the counterfeit drugs market?

Drug counterfeiting is a global scourge with alarming health and economic consequences. In her thesis entitled "Drug counterfeiting: the current situation, means of combating it and advice for patients", defended in 2015 at the University of Bordeaux, Chloé Bourgoin provides an in-depth analysis of this phenomenon and proposes strategies to combat it effectively.
Chloé Bourgoin's thesis work
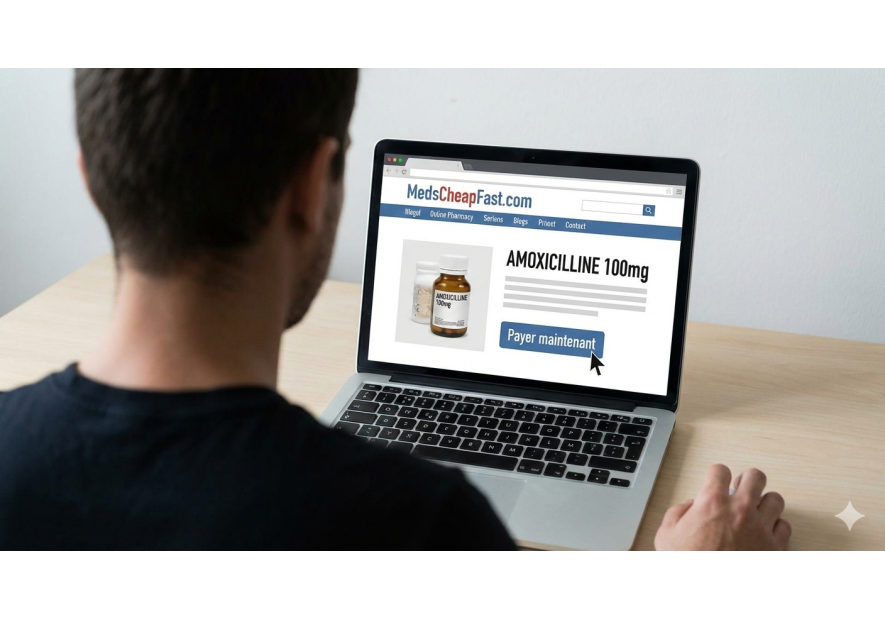
Chloé Bourgoin highlights the fact that counterfeit medicines affect all types of treatment, from life-saving drugs to comfort products. She points out that the problem is particularly worrying in developing countries, where up to 30% of medicines in circulation could be counterfeit. In industrialized countries, although the percentage is lower, the threat remains, not least because of online sales of medicines.
The author identifies several factors favoring the proliferation of counterfeit medicines:
- Supply chain vulnerabilities: Complex and poorly controlled distribution channels can allow the introduction of adulterated products.
- Unregulated online sales: Many websites offer medicines without authorization, thus evading health controls.
- Lack of public awareness: Patients are not always aware of the risks involved in buying medicines outside official channels.
Means of combating counterfeit and illegal medicines
To counter this scourge, several measures are recommended:
- Strengthening legal frameworks: The implementation of strict laws criminalizing the manufacture and distribution of counterfeit medicines is essential. The Council of Europe's MEDICRIME Convention, which came into force in 2016, is a notable example of an international treaty aimed at combating this traffic by criminalizing these acts.
- Traceability systems and security features: The adoption of technologies such as 2D barcodes (datamatrix) means that every box of medicine can be tracked throughout the supply chain, guaranteeing its authenticity. Since January 2011, the use of datamatrix has been mandatory in France for medicines, facilitating the detection of falsified products.
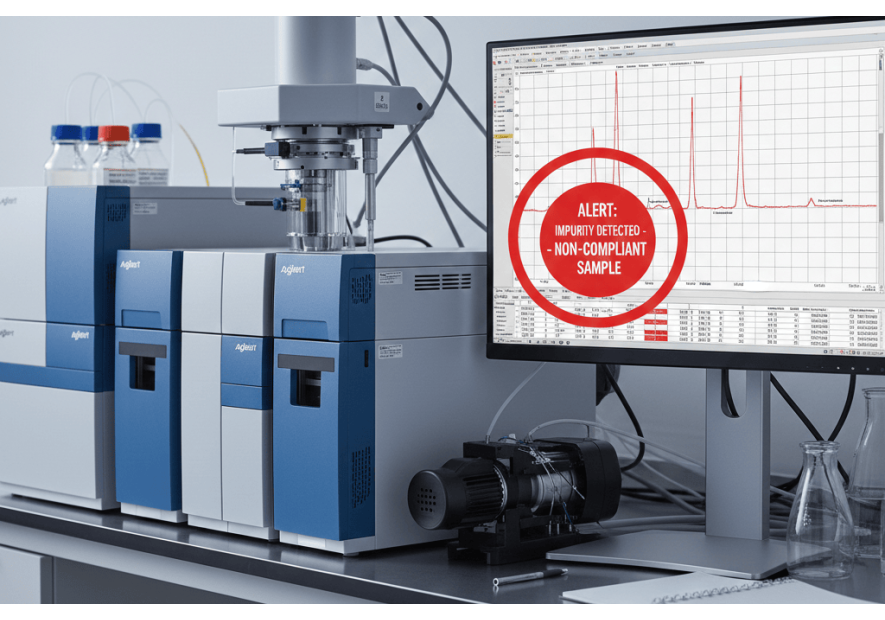
- Laboratory controls: Health authorities need the means to analyze suspect drugs. Portable tools, such as the TruScan spectrometer, enable rapid verification of product authenticity in the field.
- Public awareness and information: Informing patients about the dangers of counterfeit medicines and encouraging them to obtain their treatment only from reliable sources is crucial. Pharmacists play a key role in advising and educating their patients on this subject.
- International cooperation: As counterfeiting networks are often transnational, collaboration between countries is essential. Coordinated operations, such as Interpol's Operation Pangea, target illegal online sales and have led to the seizure of millions of counterfeit medicines.
In conclusion, Chloé Bourgoin's thesis highlights the scale of the drug counterfeiting problem and proposes a multi-dimensional approach to tackling it. A combination of legislative, technological, educational and international cooperation measures is essential to protect public health from this growing danger.
Check My Med - Participatory Science Check My Med - Participatory Science
Join Pharmanalyse in the fight against counterfeit and illegal drugs! Order our drug analysis and contribute to knowledge about the quality of medicines on the market. Our laboratory analyzes your drug, studies the data and publishes the results in a summary report accessible to the public....
Price €49.00

Leave a comment