The current situation in the fight against counterfeit medicines

Counterfeit medicines are a major threat to public health worldwide. In his thesis entitled "La contrefaçon des médicaments : les mesures de lutte européennes appliquées aux produits finis pour un laboratoire pharmaceutique fabricant et exploitant", defended in 2021 at the University of Aix-Marseille, Thomas Rochelle provides an in-depth analysis of the measures put in place within the European Union to combat this scourge, and details their practical application for pharmaceutical laboratories.
Thomas Rochelle's thesis work
Thomas Rochelle highlights the fact that, despite our best efforts, counterfeit drugs continue to grow, representing a significant threat to public health and the economy. To counter this threat, the European Union has adopted Directive 2011/62/EU, supplemented by Delegated Regulation 2016/161, aimed at strengthening the security of the pharmaceutical supply chain.
Among the key measures introduced by this regulation are :
- Anti-burglary devices : All medicinal products must be fitted with devices guaranteeing the integrity of the packaging, thus ensuring that the product has not been altered since manufacture.
- Serialization: Introduction of a unique identifier for each box of prescription drugs, enabling precise traceability throughout the distribution chain. This measure is designed to prevent the infiltration of falsified products into the legal circuit.
Implementing these measures required significant adjustments on the part of pharmaceutical companies, particularly in terms of modifying production lines to incorporate the new safety features. These adaptations generated substantial investments, estimated at between 120 and 150 million euros for all companies in the sector.
Measures to combat counterfeit and illegal medicines
In addition to European initiatives, several international actions have been taken to combat counterfeit medicines:
- MEDICRIME Convention : This international treaty of the Council of Europe criminalizes the manufacture and distribution of counterfeit medical products. It provides a legal framework for cooperation between member states, facilitating the prosecution of criminal networks involved in this traffic.
- Advanced traceability systems : Technologies such as serialization and anti-theft devices are now mandatory in Europe. Each box of medication has a unique identifier, scanned at every stage of the distribution chain, guaranteeing its authenticity before dispensing to the patient.
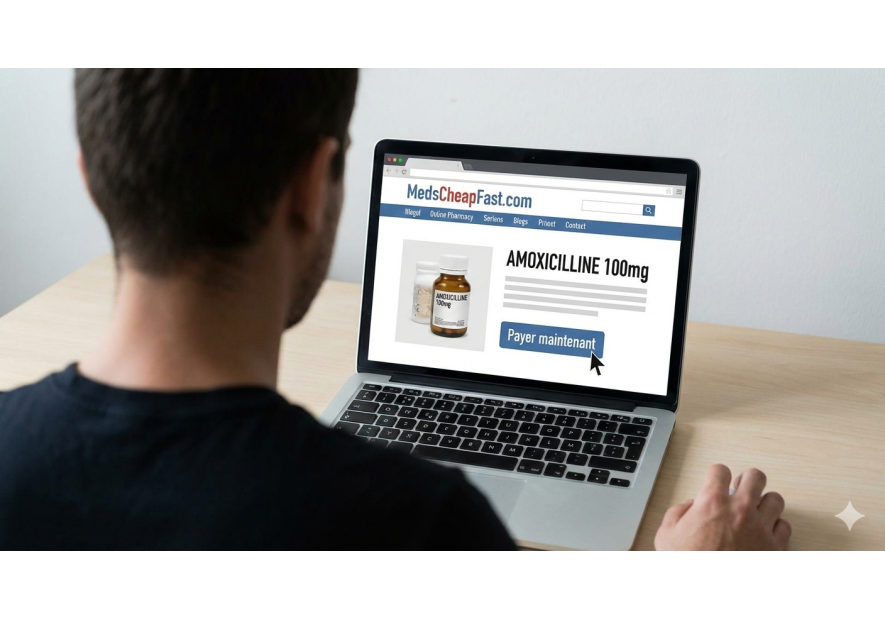
- Online monitoring : With the rise of online health services, the risk of counterfeit drugs spreading has increased. Laboratories, in collaboration with the authorities, use sophisticated tools to detect illicit offers on the Internet in real time, enabling rapid intervention to remove these products from the market.
In conclusion, Thomas Rochelle's thesis underlines the importance of a combined approach - combining strict regulation, cutting-edge technology and international cooperation - to effectively combat drug counterfeiting. Protecting public health depends on the ongoing vigilance of all those involved, from authorities to manufacturers, healthcare professionals and patients.
Check My Med - Participatory Science Check My Med - Participatory Science
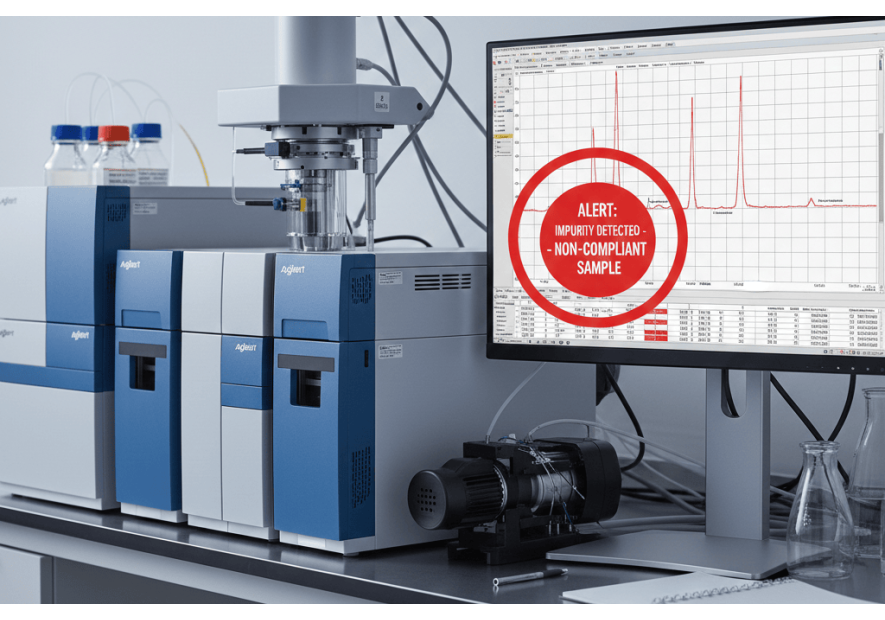
Join Pharmanalyse in the fight against counterfeit and illegal drugs! Order our drug analysis and contribute to knowledge about the quality of medicines on the market. Our laboratory analyzes your drug, studies the data and publishes the results in a summary report accessible to the public....
Price €49.00

Leave a comment